FAQ
Commonly asked questions when interested in or collaborating with different organizations to develop guidelines
Information Box Group
FAQs When Starting a Collaborative Guideline
Why collaborate when developing a guideline?
There are many reasons to pursue a collaboration when developing guideline. Opportunities to collaborate on the same topic helps minimize duplication of efforts and helps harmonize recommendations across different specialties. Guideline development panels should have representation from different stakeholders, and one of the important benefits is representation at the table. In addition, resource allocation can be shared among collaborators. Most importantly, idea generation and collaborative consensus building will allow for a more inclusive guideline.
How do I get started?
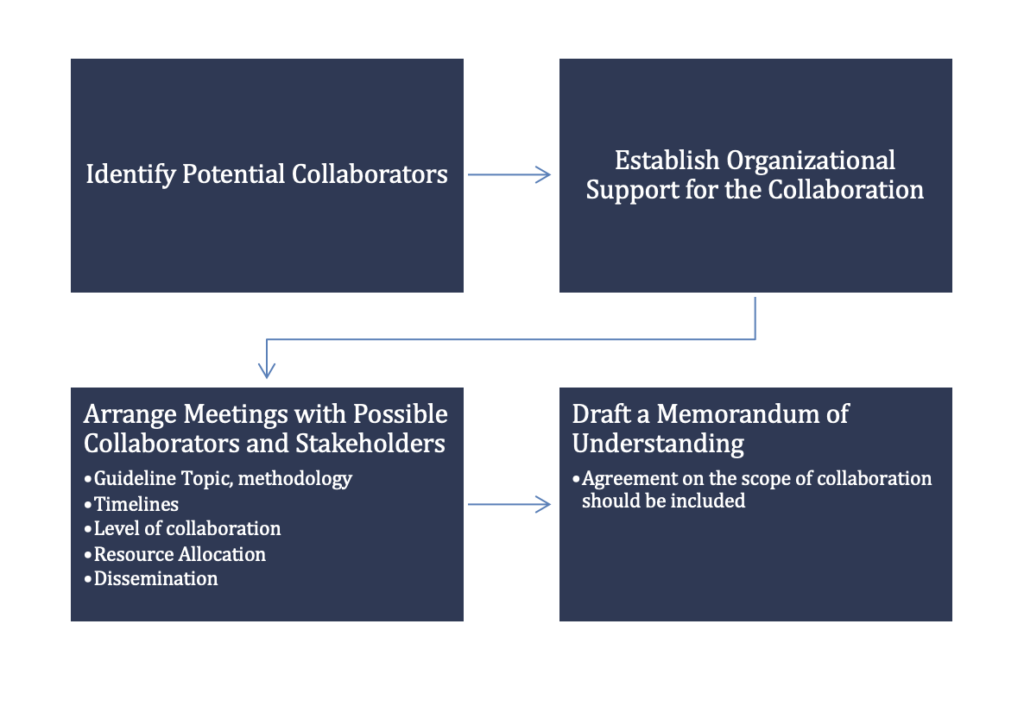
How does one begin to collaborate with other organizations with similar topics for a guideline? While most clinical practice guideline collaborations occur organically through connections and networking, some topics may not be as easy and as a guideline developer, you may need a little bit of research to begin. A good way to get started is to do scoping analysis of the topic in mind to identify possible partners. In addition, search guideline databases such as Prospero for on-going systematic reviews and GIN. Once the potential partners are identified, make sure the mission and goals of the organizations are in alignment to anticipate minimal differences in the objective setting.
Here are a few questions to consider when collaborating with another organization.
GOALS AND OUTCOMES
What are your project goals for this guideline?
The alignment of project goals is important before starting a guideline. Prior to the initial meeting with a potential collaborator, it is important to define:
- Target audience
- Important stakeholders (including patients and/or families, when possible and if applicable).
- Key research questions
- Timelines
- Guideline development methods
- Budget
These early meetings are essential to agree on all the important aspects of guideline development and managing the process. It is also important to determine if a memorandum of understanding (MOU) is needed1.
If the results do not meet organizational support, what are the next steps?
With a partnership or other collaborative relationship, most organizations will allow participation with the caveat of a final approval from its decision-making body (e.g., board of directors, approval or steering committees, or senior teams). Decisions on a disposition of the guideline from an organizations’ decision-making body can happen at the end of the process. Guideline developers should be ready for any decision, whether approved or not, and should have an action plan. For guideline collaborations with full support from the important stakeholders, the communication of organizational support and provision of funding can be described in a support document such as a methodology manual. In the event there is no support from the organization’s approval body, a contingency or backup plan should have been discussed at the onset of the project deliberations to ensure that all stakeholders agree with the plan of action in these cases.
Will the guideline be published in multiple journals or websites?
Because clinical practice guidelines are important products for many developing organizations, many guideline developers prefer publication in their own journals or websites. It is highly recommended that organizations determine and agree upon publication plans at the beginning of any collaboration. Practice guidelines are typically co-published (simultaneously published in all organization’s journals/websites) or published only in one organization’s journal, depending upon the collaboration structure. The approach for publication and dissemination may differ with a collaborative living guideline approach based on the cadence of the version updates.
ROLES AND RESPONSIBILITIES
What roles will each member organization play in the collaboration or partnership?
A MOU1 can help define roles and responsibilities for each participating organization. Deciding the roles and responsibilities before the start of the guideline is important to ensure efficient collaboration. This ensures that there is no duplication of efforts; this also helps to confirm that all aspects of the guideline roles are vetted. These roles can be distributed among different participants (e.g., subject matter experts, project manager, methodologist, medical librarian).
LEVEL OF CONTRIBUTION
What kind/level of collaboration will this project be? There are a number of ways organizations can collaborate, with varying levels of engagement, resource commitment, and responsibility. Below are two examples of collaborative guideline collaboration structures and how they differ:
- A sponsoring organization invites a collaborating organization to send subject matter expert(s) to a joint project work group2
- The sponsoring organization is responsible for all project management, guideline development costs, and retains all intellectual property; staffed by sponsoring organization
- Chair / Co-chair from sponsoring organization
- The manuscript is published in the sponsoring organization’s journal and intellectual property is not shared
- Only sponsoring organization approval is required for publication
- The invited organization may be invited to perform a limited review and potentially endorse the resulting manuscript pre-publication, but they are not required to endorse
- Sponsoring organization triggers update
- Multiple organizations agree to collaborate on a shared project work group
- All organizations share responsibility and costs for providing project management, guideline development costs; staff sharing arrangements
- Co-chairs representing each organization, typically with organizationally balanced project team representation
- Manuscripts may be co-published and intellectual property shared
- All organizations must review and endorse the final publication; if an organization does not endorse, a mechanism to publish without that organizational endorsement is included
- Any of the organizations can trigger an update
The deliverable (e.g., manuscript) from both groups can be described as a “joint guideline,” even though the collaboration structures are quite different. There are other ways organizations can collaborate, and the structure of a collaboration defines the role, resources commitment, and responsibilities of an organization. An MOU can help provide clarity to all collaborating organizations over the course of what is often a multi-year partnership to develop and update guidelines (ref the MOU paper by Murad).
RESOURCE ALLOCATION
What does each organization bring to the table?
Resource allocation is an important question to bring up. Resources beyond financial support should be considered (e.g., subject matter expertise, project managers with guideline development training, medical librarians, methodologists, systematic review platforms, etc.). Some organizations will have access to more resources than others, while other organizations may bring specialized skills to the collaboration. In some instances, organizations may have a better understanding of the guideline topic such that they may take the lead role. These resources can be discussed along with the roles each organization has to play.
How will the guideline be financed?
Guideline development is a lengthy and expensive process. Human and financial resources are needed, and some organizations have placed restrictions on guideline funding sources they will accept. This question should be prioritized in the MOU and should be discussed and documented in detail. Contingencies, fiscal year cycles (e.g., budgeting, invoicing), organizational approvals needed, etc., also need to be defined at the start of the discussions.
How will subject matter experts be selected and how many will each organization contribute?
This discussion will be based on the scope of the guideline, the structure of the guideline collaboration, and dependent on the process that is decided upon during scope development. From a project management standpoint, very large expert panels are more logistically challenging and can slow guideline development. The use of advisory panels and open comment periods can help ensure that diverse stakeholder feedback is obtained while maintaining forward progress.
TIMELINE
Is there a specific timeline that needs to be met?
Collaborating organizations should establish an initial timeline with a goal submission or publication date and an acceptable window of contingency should there be delays. If there is a certain date that a guideline must be completed, it is highly recommended that the collaborating organizations agree on that due date prior to the start of the guideline.
If the timeline is not met, what are the implications and what will be the next steps?
It is important that the collaborating organizations communicate expectations regarding timeline delays and potential consequences of those delays. If possible, the reasonable length that the project could be extended without seeking an organizational reevaluation and implications of not meeting the completion target should be clearly established at the beginning of the collaboration. Clearly defining decision points and next steps where possible can make a difficult decision much less challenging to navigate. It is also possible that one organization elects to leave a collaboration while the other collaborators agree to complete the guideline, therefore defining the processes for an organization to exit the collaboration is also important.
PROCESS
What are your organizations’ guideline development processes and are you willing to modify them if collaborating organization processes are different?
If the guideline is a shared project, all collaborating organizations will need to agree upon the development methodology to be used. If all organizations have the flexibility to do so and agree to use one process (e.g., GRADE), then the collaboration can be relatively straightforward. If organizations have different processes, a careful examination of where the processes are similar and where they diverge should be completed. A hybrid approach may be possible; however, it will require flexibility and very likely organizational approval from all collaborators. In these cases, providing additional detail regarding the guideline development process in the MOU as an addendum would be helpful to the development team. When one organization sponsors a guideline and invites collaborating organizations to a joint project, the guideline development methods of that organization will typically be used.
What are your organizations’ methods for guideline maintenance development processes and are you willing to modify them if collaborating organization processes are different?
As with the initial development of a guideline, organizations must also discuss how to maintain a guideline over time. Many guideline developers have a process for the maintenance, and there may be differences in how the update is achieved. It is important to have this discussion as part of the agreement to collaborate.
What is your organizational approval process? How long does approval typically take?
There can be wide variability in organizational approval processes and timelines. Most organizations perform a scientific review followed by a governance review to permit the name of the organization to be used on the guideline prior to journal submission, although some permit these to be concurrent processes. Organizations may have standing committees responsible for overseeing guideline development programs but convene an ad hoc subject matter expert review subcommittee for a guideline’s organizational governance review. Others have a standing committee, or a separate guideline committee, that are tasked to review each guideline prior to organizational approval. Depending upon each organization’s process, approval can take anywhere from 2-4 weeks to 6 months or more. Coordinating these approvals can be challenging, and planning discussions should occur early in a guideline collaboration.
CONFLICT OF INTEREST (COI)
What are your policies and processes regarding COIs?
Guideline COI3 policies must be developed to define significant, relevant financial, and other types of real and perceived conflicts that could call into question the integrity of the guideline. COI processes are used to gather and review COI disclosures, adjudicate the severity of COIs, and determine management strategies. These may be significantly different between organizations that wish to collaborate on a shared project, so many collaboration agreements develop and approve a joint COI policy and process. Depending upon the structure of the collaboration, the guideline may use an existing COI policy and process, or a project-specific COI policy and process may be negotiated. When one organization sponsors a guideline their COI policy and process is used for all work group members, including invited representatives.
REFERENCES
- Alam M, Getchius TS, Schünemann H, Amer YS, Bak A, Fatheree LA, Ginex P, Jakhmola P, Marsden GL, McFarlane E, Meremikwu M, Taske N, Temple-Smolkin RL, Ventura C, Burgers J, Bradfield L, O’Brien MD, Einhaus K, Kopp IB, Munn Z, Scudeller L, Schaefer C, Ibrahim SA, Kang BY, Ogunremi T, Morgan RL; Guidelines International Network (GIN) Guidelines Collaboration Working Group.. A memorandum of understanding has facilitated guideline development involving collaborating groups. J Clin Epidemiol. 2022 Apr;144:8-15. doi: 10.1016/j.jclinepi.2021.12.022. Epub 2021 Dec 16. PMID: 34923026.
- Christensen RE, Yi MD, Kang BY, Ibrahim SA, Anvery N, Dirr M, Adams S, Amer YS, Bisdorff A, Bradfield L, Brown S, Earley A, Fatheree LA, Fayoux P, Getchius T, Ginex P, Graham A, Green CR, Gresele P, Hanson H, Haynes N, Hegedüs L, Hussein H, Jakhmola P, Kantorova L, Krishnasamy R, Krist A, Landry G, Lease ED, Ley L, Marsden G, Meek T, Meremikwu M, Moga C, Mokrane S, Mujoomdar A, Newton S, O’Flynn N, Perkins GD, Smith EJ, Prematunge C, Rychert J, Saraco M, Schünemann HJ, Senerth E, Sinclair A, Shwayder J, Stec C, Tanni S, Taske N, Temple-Smolkin RL, Thomas L, Thomas S, Tonnessen B, Turner AS, Van Dam A, van Doormaal M, Wan YL, Ventura CB, McFarlane E, Morgan RL, Ogunremi T, Alam M. Development of an international glossary for clinical guidelines collaboration. J Clin Epidemiol. 2023 Jun;158:84-91. doi: 10.1016/j.jclinepi.2023.03.026. Epub 2023 Apr 3. PMID: 37019344.
- Holger J. Schünemann, Lubna A. Al-Ansary, Frode Forland, et al; for the Board of Trustees of the Guidelines International Network . Guidelines International Network: Principles for Disclosure of Interests and Management of Conflicts in Guidelines. Ann Intern Med.2015;163:548-553. [Epub 6 October 2015]. doi:10.7326/M14-1885